Uniform particle size and morphology for enhanced flowability
Efficient production and less downtime.


Efficient production and less downtime.
Enhanced tablet strength.
Smaller tablets. Larger batch size.
Optimal release of active ingredients.
Cleaner, safer production.


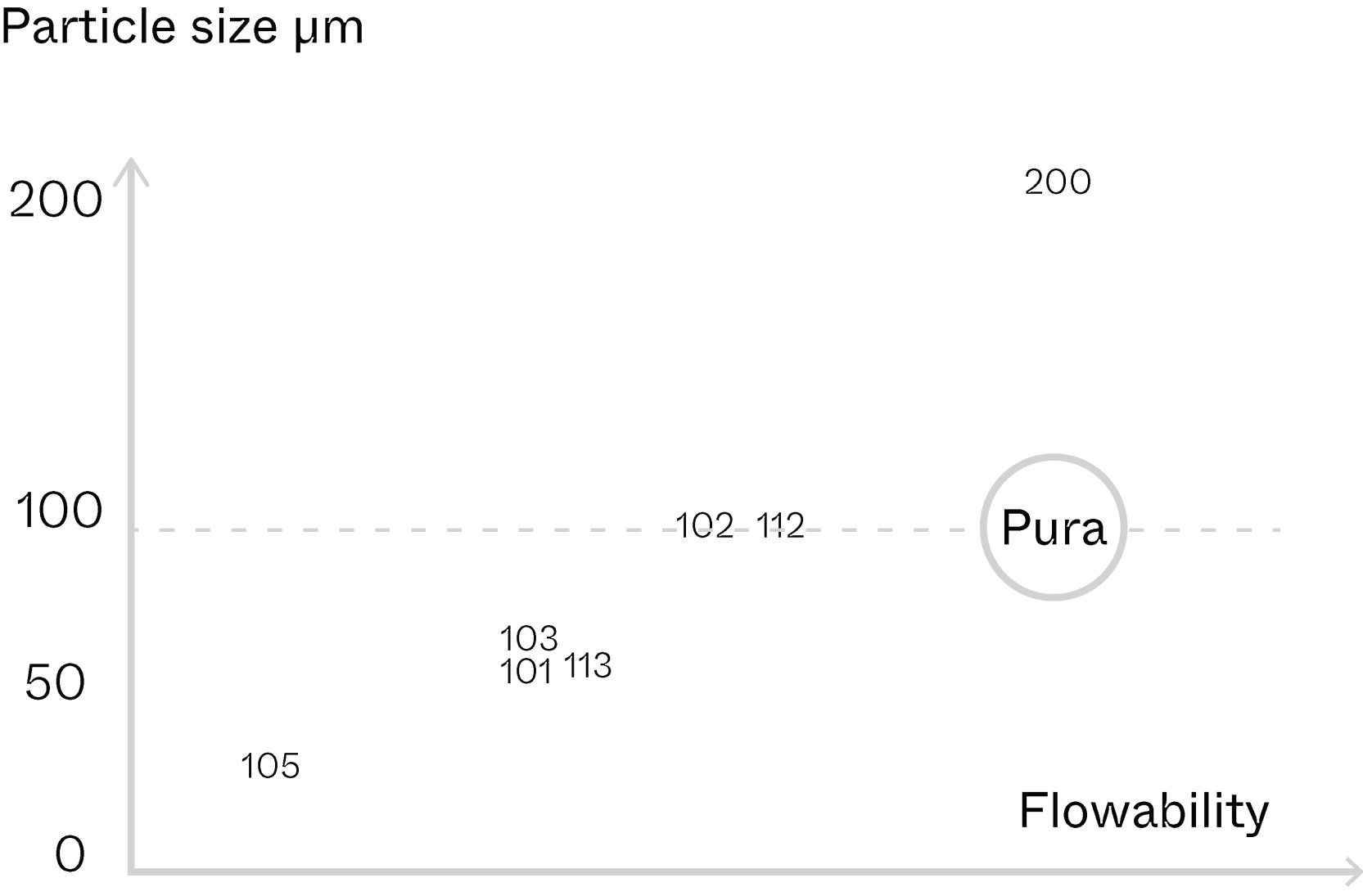
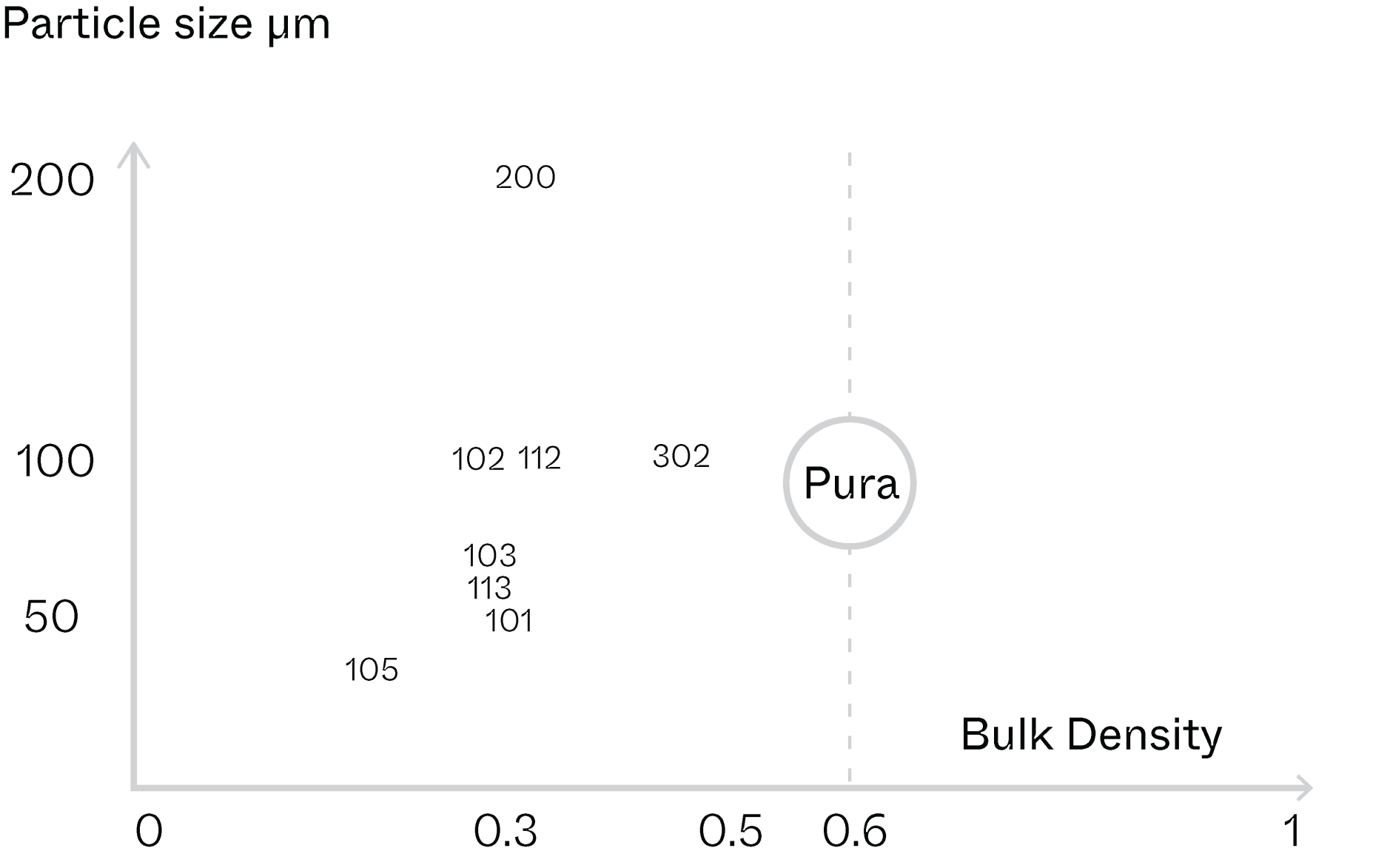
NBG MCC Pura Series: DC-95 (pre-commercial)
NBG MCC Pura Series: DC-95 (pre-commercial)
We follow GMP protocol and Pharmacopeia standards for MCC.
We comply with EU Commission Regulation No 231/2012 for food additives.
FSC certified from traceable and sustainably managed forests.
Energy-efficient and environmentally friendly process, validated through third-party assessment.
Relentless commitment to lower carbon footprint across all operations.
Supply chain security with production in Finland.


We're dedicated to finding the perfect MCC solution that fits your requirements. Reach out to us—we're here to help!